FDA Releases Data, Q&A on Potential Causes of Non-Hereditary Canine DCM
Glenn Polyn//December 28, 2022//
FDA Releases Data, Q&A on Potential Causes of Non-Hereditary Canine DCM
Glenn Polyn //December 28, 2022//
The FDA recently released a Q&A report after attending a September 2020 event where it joined scientific experts from academia, industry and veterinary medicine in a scientific forum hosted by Kansas State University to examine the potential causes of non-hereditary dilated cardiomyopathy (DCM) in dogs. During the event, scientists with research into DCM shared information, collaborate and discuss many different theories on the condition. The veterinary community, especially veterinary nutritionists and veterinary cardiologists and other specialists; industry and academia, continue to examine this issue to help determine what factors may be contributing to the heart conditions observed and reported to FDA.
The FDA has compiled the following questions and answers to address those frequently asked by pet owners and veterinarians.
1. What is canine dilated cardiomyopathy (DCM) and how does non-hereditary DCM differ from the genetic form?
DCM is a disease of a dog’s heart muscle and results in an enlarged heart. As the heart and its chambers become dilated, it becomes harder for the heart to pump, and heart valves may leak, which can lead to a buildup of fluids in the chest and abdomen (congestive heart failure).
Historically, DCM has been primarily linked to a genetic predisposition in certain breeds, but emerging science appears to indicate that non-hereditary forms of DCM occur in dogs as a complex medical condition that may be affected by the interplay of multiple factors such as genetics, underlying medical conditions, and diet. Aspects of diet that may interact with genetics and underlying medical conditions may include nutritional makeup of the ingredients and how dogs digest them, ingredient sourcing, processing, formulation, and/or feeding practices.
Reports from veterinary cardiologists demonstrate some good results in improving heart function in non-hereditary DCM cases, unlike genetic forms of DCM, with appropriate veterinary treatment and dietary modification, when caught early in the progression of the disease.
2. Why is FDA focused on potential dietary causes of non-hereditary DCM?
While non-hereditary DCM appears to be caused by a confluence of multiple factors, FDA is a regulatory agency and has regulatory authority over animal food, including pet food, thus the reason for the agency’s focus on diet as a potential contributor. There is no public health agency that tracks animal health in the same way that the Centers for Disease Control and Prevention tracks human health, therefore FDA has called on the veterinary and academic communities, as well as industry, to contribute research on various aspects of non-hereditary DCM. Many representatives from these sectors participated in the recent KSU scientific forum and several agreed to publicly share their abstracts and/or presentations.
3. What is FDA doing to better understand non-hereditary DCM cases?
Our veterinarians, animal nutritionists, epidemiologists and pathologists have been working with veterinary cardiologists and nutritionists from academia, industry and private practice to better understand the clinical presentation of the cases and potential ties to diet, such as bioavailability of critical nutrients and how well a dog digests these nutrients. FDA scientists also presented at the KSU symposium on a subset of dogs that had fully or partially recovered from DCM with diet change and veterinary care.
4. What additional information would help further understanding of non-hereditary DCM?
FDA is encouraged by the response of veterinary cardiologists, veterinary nutritionists, academia and industry in delving into this issue and we encourage other scientists to take part. As we look further into the role that diet may play in these cases, we hope to explore additional avenues of inquiry such as formulation, nutrient bioavailability, ingredient sourcing, and diet processing to determine if there are any common factors. We have asked pet food manufacturers to share diet formulation information, which could substantially benefit our understanding of the role of diet in the development of non-hereditary DCM. Formulation data shared with the FDA will be kept confidential.
5. How many reports of DCM has FDA received?
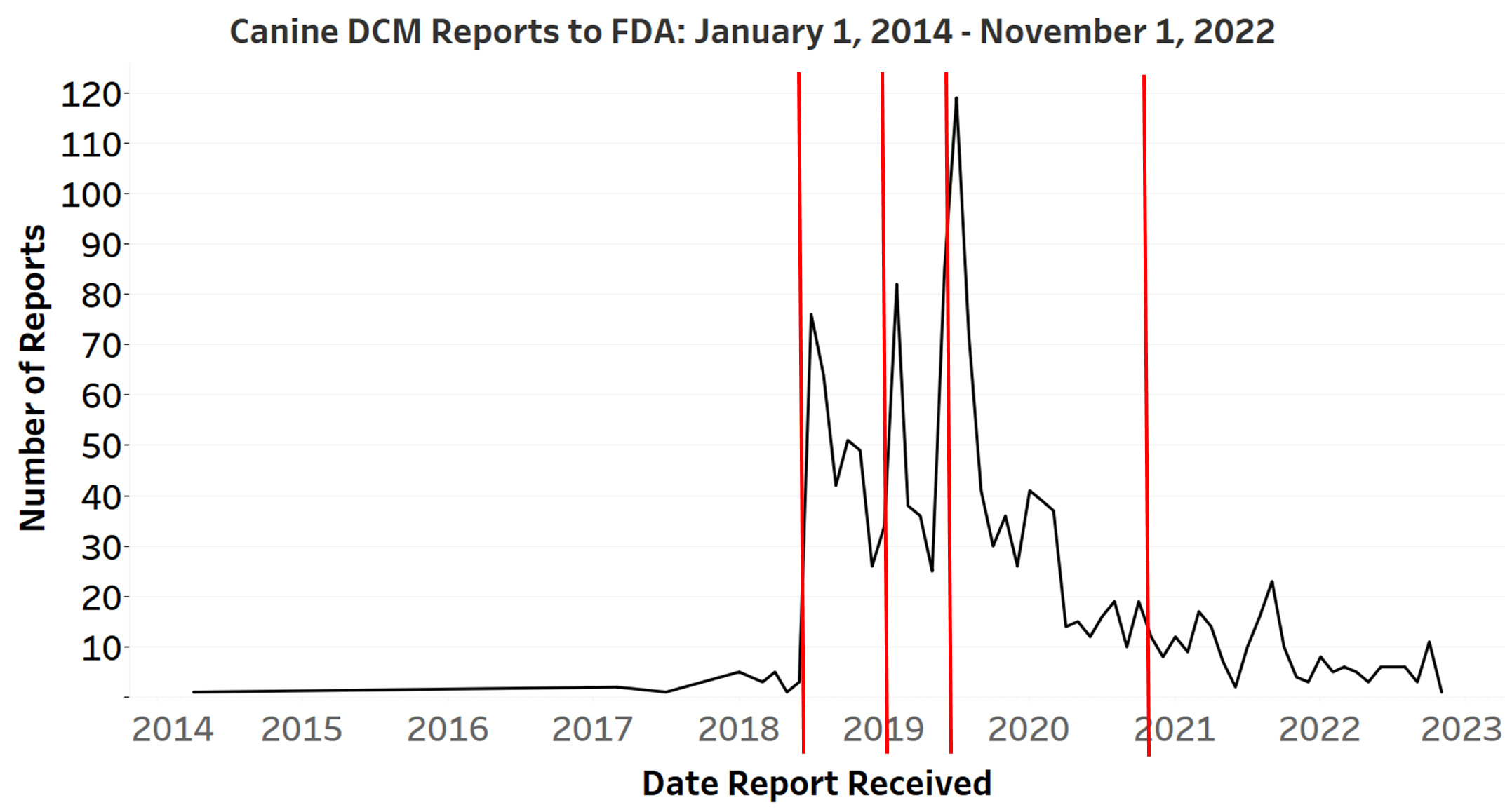
Since the agency’s last public communication on the investigation in 2020, FDA continues to receive reports of DCM diagnoses in dogs, but at lower levels than experienced from 2018 through 2020 (see figure and table below). Some of the reports involve more than one individual animal from a single household. While FDA has followed up on a subset of these reports, the agency is unable to investigate every report to verify or confirm the reported information.
Over time, FDA has published information about the number of reports the agency has received. These communications have varied in their content – sometimes including cats, as well as dogs, or only including those reports that FDA had confirmed with records from a veterinary cardiologist. In December 2022, as part of a routine update to Congress, the FDA compiled information on canine DCM reports submitted to the agency. FDA has opted to share that information here for public awareness.
The graph shows the number of reports submitted to FDA since 2014 and indicates the dates on which FDA issued public updates on the investigation. The table that follows shows the number of reports of DCM in dogs submitted between previous public communications from FDA, up to November 1, 2022.
Number of reports submitted to FDA of DCM in dogs:
| Jan. 1, 2014 – Nov 30, 2018 | Dec 1, 2018 – Apr 30, 2019 | May 1, 2019 – July 31, 2020 | Aug 1, 2020 – Nov 1, 2022 | Total: Jan 1, 2014 – Nov 1, 2022 |
|---|---|---|---|---|
| 329 | 190 | 608 | 255 | 1,382 |
According to the FDA, it is typical for it to receive a short-term increase in reports after issuing public updates on a pet health issue. In this case, upticks in the number of DCM reports to FDA tend to happen after FDA issues public updates on the DCM issue. Public updates are indicated by the red lines in the graph above.