USDA Grants Zoetis Inc. a License for Cytopoint
Pet Age Staff //December 22, 2016//
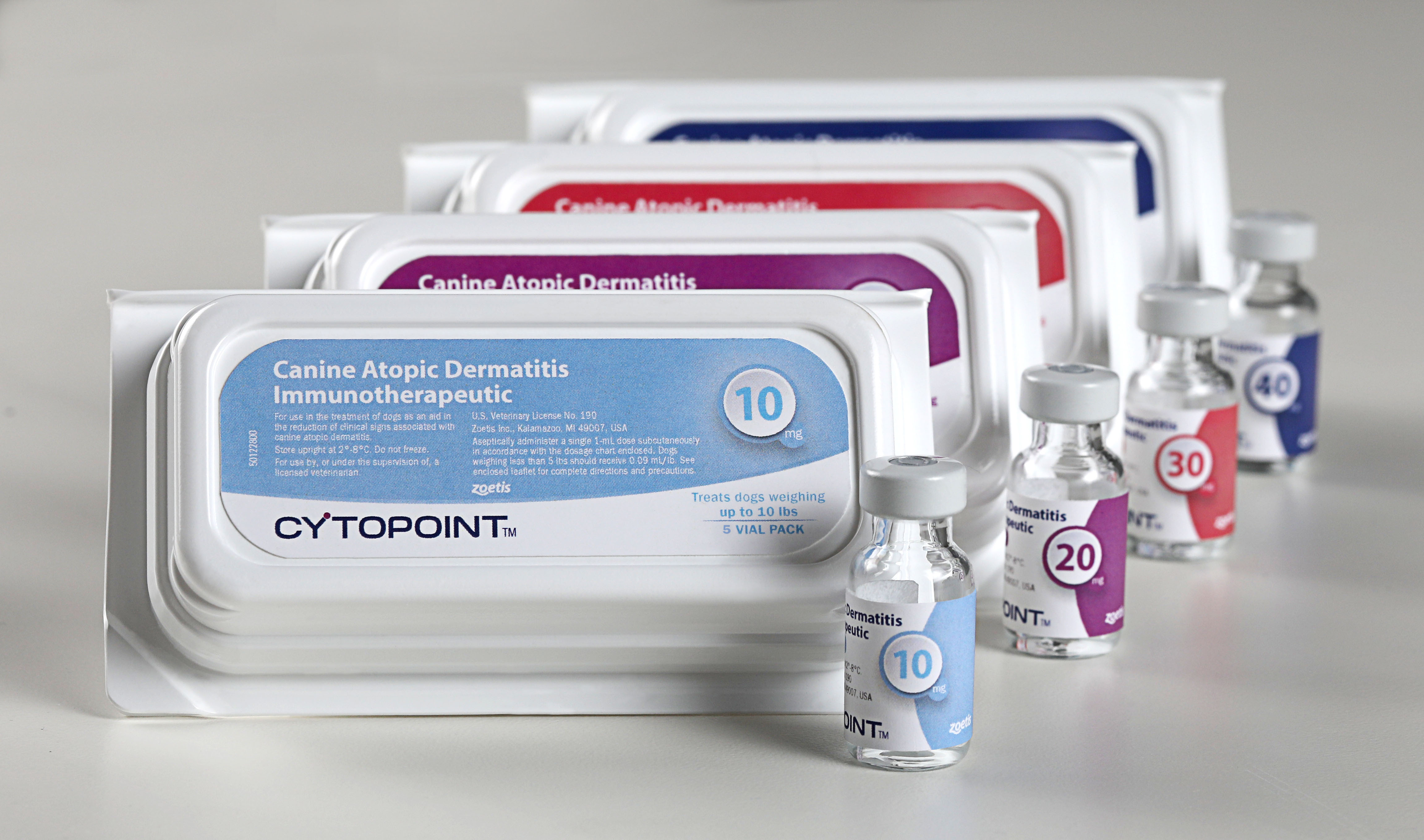
Zoetis Inc. announced that the U.S. Department of Agriculture (USDA) granted the company a license for CYTOPOINT, the first monoclonal antibody (mAb) therapy approved to help provide sustained control of the clinical signs associated with atopic dermatitis in dogs. CYTOPOINT targets and neutralizes interleukin-31 (IL-31), a key protein involved in triggering itch in dogs. It provides fast, effective relief of itching—the hallmark sign of the allergic skin condition atopic dermatitis in dogs—and offers the sustained efficacy and convenience of one injection every four to eight weeks. CYTOPOINT helps improve the long-term quality of life for dogs suffering from atopic dermatitis and eases the related frustration and concern of their owners. It is now available to all veterinarians in the United States.
“As the owner of allergic pets, I understand the frustration that my clients feel, and as a person with allergies myself, I understand what my patients feel,” said Laura Stokking, PhD, DVM, DACVD, Veterinary Specialty Hospital, San Diego, California. “With CYTOPOINT, in a single injection, we now have an excellent opportunity to help control the itch without leading to any secondary signs that can be more difficult to manage than the itch itself.”
“CYTOPOINT results from our acquiring a deeper scientific understanding of the causes of allergic skin conditions in dogs at the molecular level and developing novel, targeted, effective treatments based on these new insights,” said Dr. Catherine Knupp, executive vice president and president of Research and Development at Zoetis. “Veterinarians have told us that allergic dogs and their owners have a variety of needs and we are proud to offer them two innovative solutions with CYTOPOINT and with our oral tablet therapy APOQUEL. These first-in-class medicines give veterinarians effective, safe options to customize atopic dermatitis treatment for canine patients, and I am very proud of the breakthrough treatments our Zoetis team has developed.”
APOQUEL (oclacitinib tablet) is the first Janus kinase inhibitor approved by the U.S. Food and Drug Administration for veterinary use to provide fast and safe itch relief for dogs at least 12 months of age that have symptoms associated with allergic dermatitis triggered by food, fleas or contact allergens, as well as atopic dermatitis.